Dec 13, 2023Therefore, it is a strong electrolyte. 2. HC2H3O2 (Acetic Acid): This is a weak acid, which means it does not completely ionize in water. Therefore, it is a weak electrolyte. 3. NH3 (Ammonia): This is a weak base, which means it does not completely ionize in water. Therefore, it is a weak electrolyte. 4. KCl (Potassium Chloride): This is a salt
Multiple Choice Questions in Science: Chapter 7 : ACIDS, BASES AND SALTS
As with acids, there are only a few strong bases, which are also listed in Table 10.2 “Strong Acids and Bases (All in Aqueous Solution)”. If an acid is not listed in Table 10.2 “Strong Acids and Bases (All in Aqueous Solution)”, it is likely a weak acid that is far less than 100% ionized in aqueous solution. Similarly, a weak base is a

Source Image: study.com
Download Image
KCl is a strong electrolyte and the bulb is very bright. Acetic acid is a weak electrolyte, and although the image may not show it, if the concentrations are the same, the light is dimmer than for the KCl. Exercise 3.4.1. Both salt (NaCl) and table sugar, glucose (C 6 H 12 O 6) dissolve in water.

Source Image: numerade.com
Download Image
PG.CHEMEASY: Benzoic acid-weak acid-stronger than acetic acid weaker than formic acid. Sep 27, 2022The issue is similar with bases: a strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base. There are very few strong bases (Table \(\PageIndex1\)); any base not listed is a weak base. All strong bases are OH – compounds.

Source Image: reddit.com
Download Image
Is Hc2h3o2 A Strong Or Weak Electrolyte
Sep 27, 2022The issue is similar with bases: a strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base. There are very few strong bases (Table \(\PageIndex1\)); any base not listed is a weak base. All strong bases are OH – compounds. Weak Electrolyte Examples. HC 2 H 3 O 2 (acetic acid), H 2 CO 3 (carbonic acid), NH 3 (ammonia), and H 3 PO 4 (phosphoric acid) are all examples of weak electrolytes. Weak acids and weak bases are weak electrolytes. In contrast, strong acids, strong bases, and salts are strong electrolytes. Note a salt may have low solubility in water, yet
Why is the answer A instead of C? : r/chemhelp
Molecular Examples. CH 3 OH – methyl alcohol. C 2 H 5 OH – ethyl alcohol. C 6 H 12 O 6 – glucose. Electrolytes are chemicals that break into ions in water. What strong, weak, and non-electrolytes are and examples of each type. SOLVED: Lyte, a weak electrolyte, or a nonelectrolyte: Solutes Formula Hydrochloric HCl acid Calcium hydroxide Acetic acid Ca(OH)2 H: CCOOH Propylamine CH3CH2CH2NH2 Sodium bromide NaBr Butanol CH3OH C6H12O6 Glucose
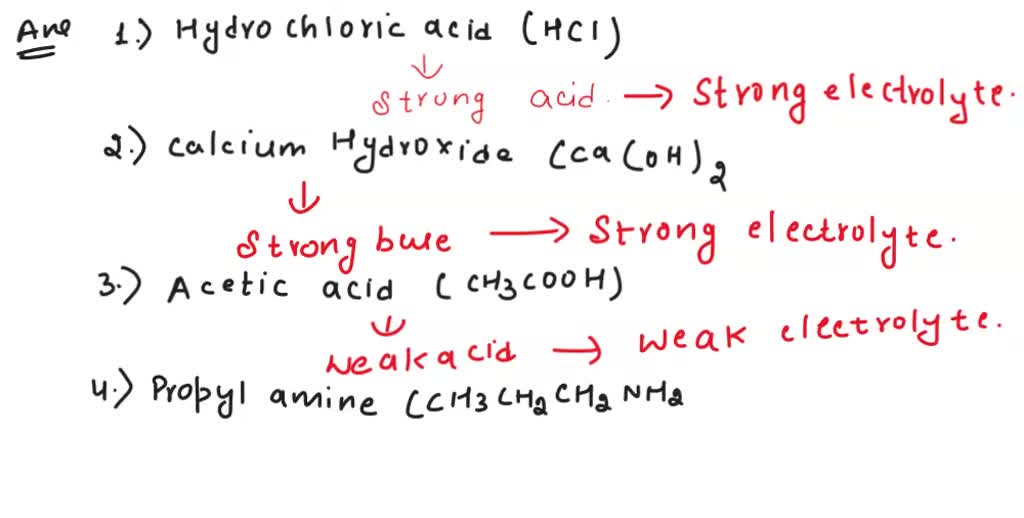
Source Image: numerade.com
Download Image
Acid–Base Equilibrium in Water | Read Chemistry Molecular Examples. CH 3 OH – methyl alcohol. C 2 H 5 OH – ethyl alcohol. C 6 H 12 O 6 – glucose. Electrolytes are chemicals that break into ions in water. What strong, weak, and non-electrolytes are and examples of each type.
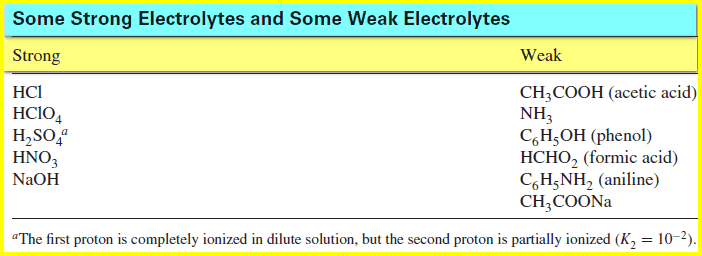
Source Image: readchemistry.com
Download Image
Multiple Choice Questions in Science: Chapter 7 : ACIDS, BASES AND SALTS Dec 13, 2023Therefore, it is a strong electrolyte. 2. HC2H3O2 (Acetic Acid): This is a weak acid, which means it does not completely ionize in water. Therefore, it is a weak electrolyte. 3. NH3 (Ammonia): This is a weak base, which means it does not completely ionize in water. Therefore, it is a weak electrolyte. 4. KCl (Potassium Chloride): This is a salt

Source Image: science-by-manojsir.blogspot.com
Download Image
PG.CHEMEASY: Benzoic acid-weak acid-stronger than acetic acid weaker than formic acid. KCl is a strong electrolyte and the bulb is very bright. Acetic acid is a weak electrolyte, and although the image may not show it, if the concentrations are the same, the light is dimmer than for the KCl. Exercise 3.4.1. Both salt (NaCl) and table sugar, glucose (C 6 H 12 O 6) dissolve in water.

Source Image: chemisfast.blogspot.com
Download Image
Acid–Base Equilibrium in Water | Read Chemistry Science. Chemistry. Chemistry questions and answers. 1. a: Acetic acid (HC2H3O2) is a weak electrolyte. What substances are present in HC2H3O2 (aq)? b: Chloric acid (HClO3) is a strong electrolyte. What substances are present in HClO3 (aq)? 2. Identify each of the following substances as a strong electrolyte, weak electrolyte, or nonelectrolyte: a.
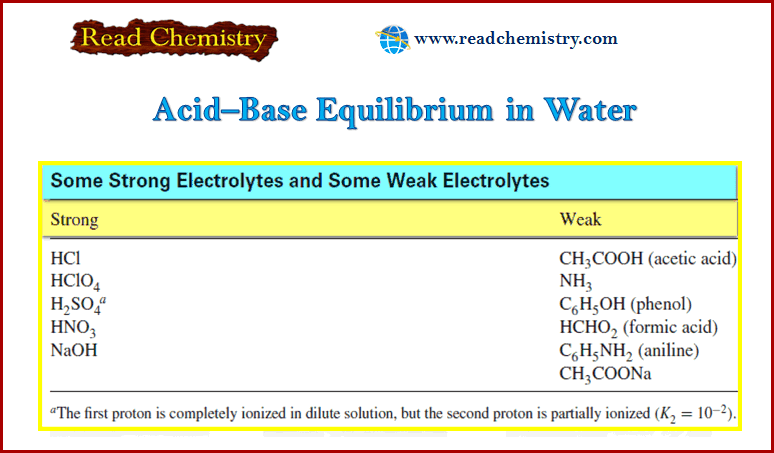
Source Image: readchemistry.com
Download Image
EduMission: Chemistry Form 4: Chapter 7 – How to Differentiate Between Strong Acid and Weak Acid Sep 27, 2022The issue is similar with bases: a strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base. There are very few strong bases (Table \(\PageIndex1\)); any base not listed is a weak base. All strong bases are OH – compounds.

Source Image: cikguwong.blogspot.com
Download Image
SOLVED: 5. Mixtures of a weak and a strong electrolyte; a) What is the pH in 0.3 M solution of KOOCCH? Acetic acid has pKa = 4.76. Plot pH vs. the total Weak Electrolyte Examples. HC 2 H 3 O 2 (acetic acid), H 2 CO 3 (carbonic acid), NH 3 (ammonia), and H 3 PO 4 (phosphoric acid) are all examples of weak electrolytes. Weak acids and weak bases are weak electrolytes. In contrast, strong acids, strong bases, and salts are strong electrolytes. Note a salt may have low solubility in water, yet

Source Image: numerade.com
Download Image
Acid–Base Equilibrium in Water | Read Chemistry
SOLVED: 5. Mixtures of a weak and a strong electrolyte; a) What is the pH in 0.3 M solution of KOOCCH? Acetic acid has pKa = 4.76. Plot pH vs. the total As with acids, there are only a few strong bases, which are also listed in Table 10.2 “Strong Acids and Bases (All in Aqueous Solution)”. If an acid is not listed in Table 10.2 “Strong Acids and Bases (All in Aqueous Solution)”, it is likely a weak acid that is far less than 100% ionized in aqueous solution. Similarly, a weak base is a
PG.CHEMEASY: Benzoic acid-weak acid-stronger than acetic acid weaker than formic acid. EduMission: Chemistry Form 4: Chapter 7 – How to Differentiate Between Strong Acid and Weak Acid Science. Chemistry. Chemistry questions and answers. 1. a: Acetic acid (HC2H3O2) is a weak electrolyte. What substances are present in HC2H3O2 (aq)? b: Chloric acid (HClO3) is a strong electrolyte. What substances are present in HClO3 (aq)? 2. Identify each of the following substances as a strong electrolyte, weak electrolyte, or nonelectrolyte: a.