Jul 18, 2022The charge on an atom is related to its valence electrons or oxidation state. An atom of an element is most stable when its outer electron shell is completely filled or half-filled. The most common charges are based on maximum stability for the atom. However, other charges are possible.
Binding Energy and Free Energy of Calcium Ion to Calmodulin EF-Hands with the Drude Polarizable Force Field | ACS Physical Chemistry Au
So calcium ion would be Ca ++ and have a positive charge of 2 x charge of an electron = 2 x 1.6 x 10 -19 Coloumb. Each chlorine ion would be Cl – with a negative charge of one electron = 1.6 x 10 -19 C. 🙂 Upvote • 0 Downvote Add comment Report Still looking for help? Get the right answer, fast. Ask a question for free

Source Image: chemistry-europe.onlinelibrary.wiley.com
Download Image
Science Chemistry Chemistry questions and answers Calcium’s atomic number is 20. It forms ions with 18 electrons. What is the electrical charge of a calcium ion? 0 -2 O +2 0 -1 O +1 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: reddit.com
Download Image
Calcium Nitrate Ca(NO3)2 – Structure, Properties, Uses, FAQs May 26, 2023During the chemical reaction, calcium loses these 2 electrons and achieves the nearest noble gas configuration to become stable. And as the Calcium (Ca) loses 2 electrons, it forms Ca 2+ ion. Hence the ionic charge of Calcium (Ca) is 2+. I hope you have understood the reason behind the 2+ charge of calcium. Check out some other related topics
Source Image: windows2universe.org
Download Image
What Is The Electrical Charge Of A Calcium Ion
May 26, 2023During the chemical reaction, calcium loses these 2 electrons and achieves the nearest noble gas configuration to become stable. And as the Calcium (Ca) loses 2 electrons, it forms Ca 2+ ion. Hence the ionic charge of Calcium (Ca) is 2+. I hope you have understood the reason behind the 2+ charge of calcium. Check out some other related topics The charge close charge (electrical) An imbalance of electrons and protons in a material. An excess of electrons results in negative charge, a deficit of electrons results in positive charge. of
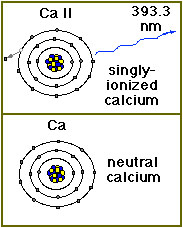
Calcium Ions (Ca II K) in the Sun’s Atmosphere
Answer link A +2 oxidation state, typically. “Ca” generally loses two electrons from its outer shell to form a “Ca”^ (2+) ion. Charge on “Ca”^ (2+) ion is “q” = 2 cancel”positive charges” × (1.602 × 10^-19\ “C”)/cancel”proton” = 3.214 × 10^-19\ “C” Ion Formation When an atom gains or loses electrons they become electrically charged particles called Ions Metals tend to lose electrons Positive ions. – ppt download

Source Image: slideplayer.com
Download Image
Ca 2+ Electron Configuration (Calcium Ion) – YouTube Answer link A +2 oxidation state, typically. “Ca” generally loses two electrons from its outer shell to form a “Ca”^ (2+) ion. Charge on “Ca”^ (2+) ion is “q” = 2 cancel”positive charges” × (1.602 × 10^-19\ “C”)/cancel”proton” = 3.214 × 10^-19\ “C”

Source Image: youtube.com
Download Image
Binding Energy and Free Energy of Calcium Ion to Calmodulin EF-Hands with the Drude Polarizable Force Field | ACS Physical Chemistry Au Jul 18, 2022The charge on an atom is related to its valence electrons or oxidation state. An atom of an element is most stable when its outer electron shell is completely filled or half-filled. The most common charges are based on maximum stability for the atom. However, other charges are possible.

Source Image: pubs.acs.org
Download Image
Calcium Nitrate Ca(NO3)2 – Structure, Properties, Uses, FAQs Science Chemistry Chemistry questions and answers Calcium’s atomic number is 20. It forms ions with 18 electrons. What is the electrical charge of a calcium ion? 0 -2 O +2 0 -1 O +1 This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Source Image: byjus.com
Download Image
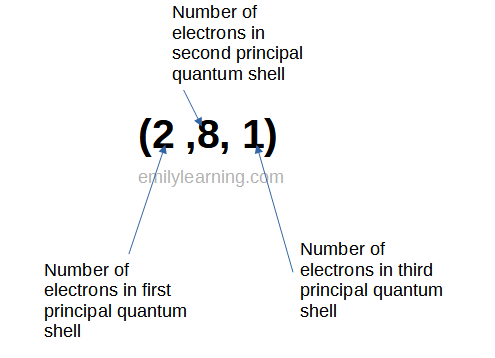
Write Electronic Configuration of atoms and ions – for O Level Chemistry – Emily Learning A calcium ion is written as [Ca]^(2+). Calcium atoms tend to lose 2 electrons from its valence shell to have a full shell of 8 electrons. For this reason, calcium’s charge is 2^+. To indicate the loss of 2 electrons, we write it as: [Ca]^(2+) where: [ ] = indicates that the atom is an ion Ca = the element 2+ = the charge of calcium after losing 2 electrons Note: When an atom loses electrons
Source Image: emilylearning.com
Download Image
Calcium Atom and Ion « KaiserScience May 26, 2023During the chemical reaction, calcium loses these 2 electrons and achieves the nearest noble gas configuration to become stable. And as the Calcium (Ca) loses 2 electrons, it forms Ca 2+ ion. Hence the ionic charge of Calcium (Ca) is 2+. I hope you have understood the reason behind the 2+ charge of calcium. Check out some other related topics

Source Image: kaiserscience.wordpress.com
Download Image
Sodium, Potassium and Calcium Ions – Curriculum Press The charge close charge (electrical) An imbalance of electrons and protons in a material. An excess of electrons results in negative charge, a deficit of electrons results in positive charge. of

Source Image: curriculum-press.co.uk
Download Image
Ca 2+ Electron Configuration (Calcium Ion) – YouTube
Sodium, Potassium and Calcium Ions – Curriculum Press So calcium ion would be Ca ++ and have a positive charge of 2 x charge of an electron = 2 x 1.6 x 10 -19 Coloumb. Each chlorine ion would be Cl – with a negative charge of one electron = 1.6 x 10 -19 C. 🙂 Upvote • 0 Downvote Add comment Report Still looking for help? Get the right answer, fast. Ask a question for free
Calcium Nitrate Ca(NO3)2 – Structure, Properties, Uses, FAQs Calcium Atom and Ion « KaiserScience A calcium ion is written as [Ca]^(2+). Calcium atoms tend to lose 2 electrons from its valence shell to have a full shell of 8 electrons. For this reason, calcium’s charge is 2^+. To indicate the loss of 2 electrons, we write it as: [Ca]^(2+) where: [ ] = indicates that the atom is an ion Ca = the element 2+ = the charge of calcium after losing 2 electrons Note: When an atom loses electrons